Transfuse the right product to the right patient
A complete and easy-to-use system that complies with EU Directives on blood safety and quality, and the transfusion guidelines of many countries
 |
TDBloodBank manages the transfusion request workflow, from stock entry, sample reception and testing, and crossmatch and antibody screening, to post-transfusion tracking and audit. It is a highly customizable solution which can be configured to meet all blood management and transfusion needs. |



TDBloodBank is highly customizable and can be configured to meet all blood management and transfusion needs. It can easily be adapted to suit local working practices and legislation, always with patient safety as a priority.
- Compliance with European Directives on blood safety and quality
- Positive patient identity (optional Safetrak Pocket module at the bedside)
- Blood group and compatibility testing
- Antibody screening
- Positive patient identity (optional Safetrak Pocket module at the bedside)
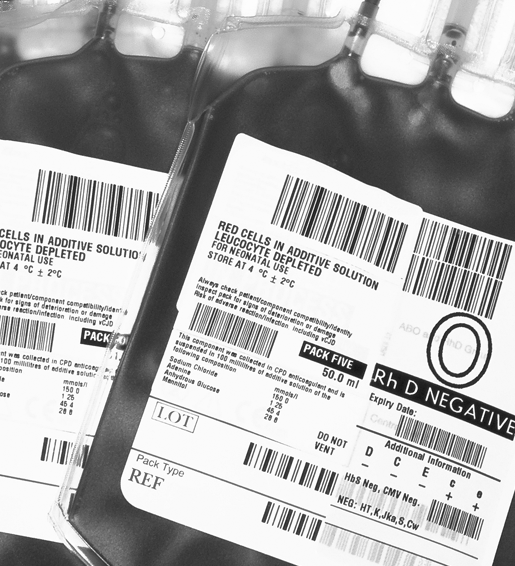
- Manages stocks efficiently and safely
- Time out of fridge monitoring
- Pack quarantine before release to stock
- Remote fridge access control (optional Safetrak Fridge module)
- Supports ISBT128 product labeling standards
- Safer, faster stock entry
- Easier pack identification at all stages in the process

- Safer, faster stock entry
- Manages non-standard workflows, such as those for patients with:
- Transitional blood groups
- Rare phenotypes like Bombay/Para-Bombay
- Transitional blood groups
TDBloodBank optimizes workflows for faster turnaround times on sample testing, and faster and safer access to transfusion:
- Sample identification and reception
- Sample reception procedure
- Sample reception procedure
- Sample testing
- Use of mother’s sample for neonates
- Automatic comparison with previous results
- Alerts in the event of blood group mismatches
- Optional setting to prevent pack allocation before testing is complete
- Interfaces with the major blood-group typing instruments
- Blood group confirmation
- Consolidated results at patient level
- Single or double determination
- Consolidated results at patient level
- Antibody screening
- Automatic checks for available patient samples
- Date/time of previous transfusions determines if a new screen is required
- Automatic checks for available patient samples
- Compatibility and crossmatch
- Configurable compatibility tables for ABO, Rhesus, Phenotype, Kell and Bombay
- Essential patient information displayed at all times
- Specific transfusion requirements, such as irradiated blood or CMV negative
- Electronic issue (optional crossmatch) in line with international guidelines
- Up to four methods of manual crossmatch where Electronic Issue is not permitted or suitable
- Interfaces with the major automated crossmatch instruments
At every stage of the process, TDBloodBank offers comprehensive traceability of user and system actions:
- Quarantine at stock entry
- Serological or electronic crossmatch
- Stock update
- Product issue, including stat issue
- Return to stock
- Fate of donation